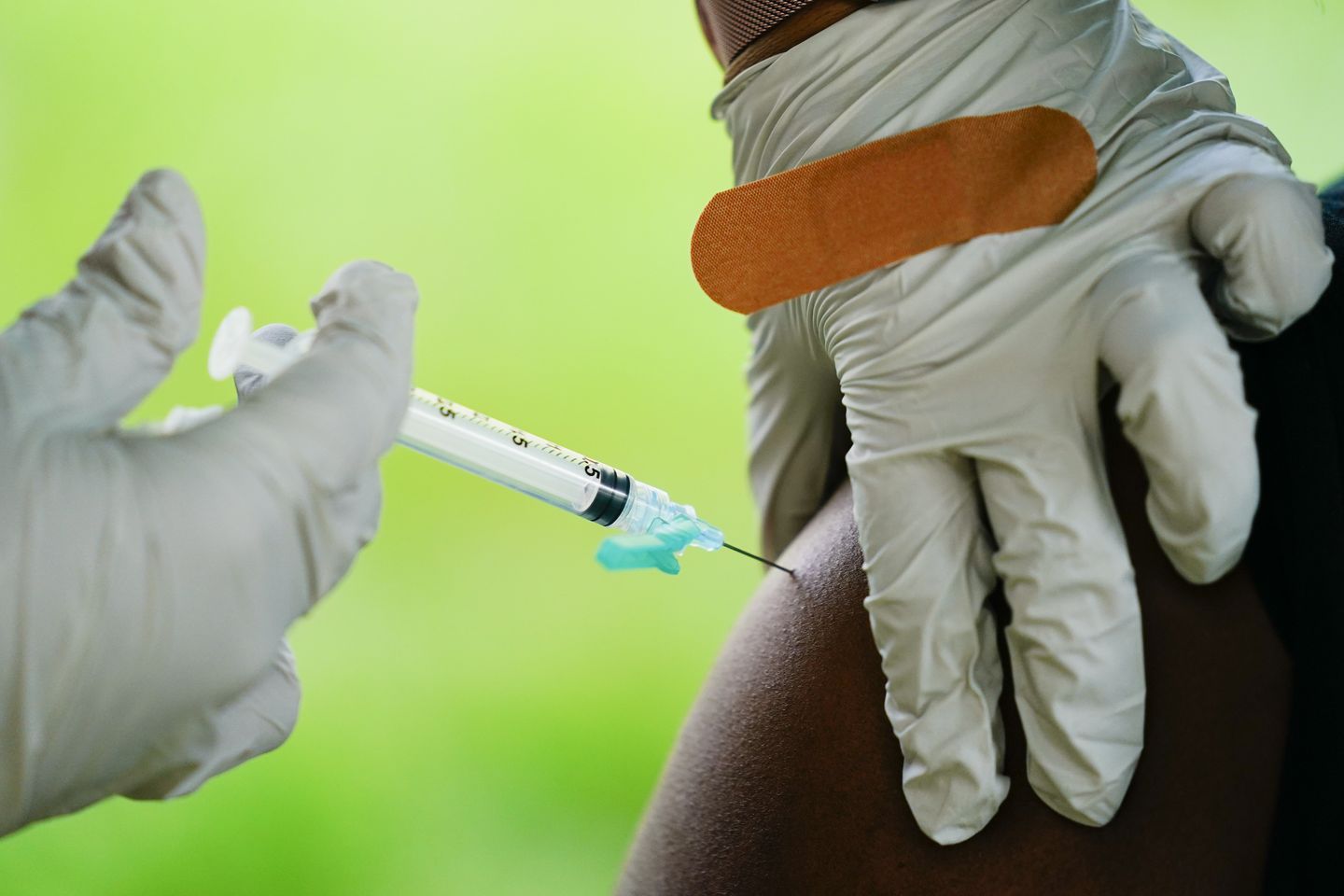
Print By Joseph Clark - The Washington Times - Friday, March 10, 2023 House investigators are probing whether the Biden administration pressured the Food and Drug Administration to fast-track approval for a leading COVID-19 jab required to implement and enforce vaccine mandates. The House COVID-19 panel is demanding that the FDA turnover details of its review of Pfizer/BioNTech’s COVID-19 vaccine. In a letter to FDA making the demands, lawmakers unveiled internal emails they say give the appearance that the Biden administration “may have bypassed, wrongly compressed, and possibly compromised the longstanding process for awarding a full biologics approval” to the vaccine.
” In a letter Friday to FDA Commissioner Dr. Robert Mr. Califf, the lawmakers also allege the quick approval timeline set by the administration “may not have been to save lives, but concernedly to provide cover for implementing and enforcing vaccine mandates across the country.
” The letter was signed by Republican lawmakers including House Oversight and Accountability Select Subcommittee on the Coronavirus Chairman Brad Wenstrup of Ohio and committee’s Health Care and Financial Services Chairwoman Lisa McClain of Michigan and Subcommittee on Health Care and Financial Services Chairwoman Lisa McClain of Michigan. The FDA said it had received the letter and would respond directly to the subcommittee. The White House and Pfizer did not immediately respond to a request for comment.
In the letter, the lawmakers reveal portions of an email from Dr. Marion Gruber, the former Director of the FDA’s Office of Vaccines Research and Review (OVVR) in which she expressed concerns to her colleagues with the Biden administration’s timeline for approving the vaccine. According to the lawmakers, Pfizer/BioNTech submitted its application for full FDA approval of its Comirnaty vaccine in May of 2021.
The FDA granted the application priority review the next month, and set a “public goal” of January 2022 for approving the vaccine, while privately setting a goal of approving the shot by Sept. 15, 2021. Dr.
Gruber wrote to colleagues on July 19, 2021, warning that the review timeline “cannot be compressed further” beyond the September deadline, noting that the timeline was less than half of that normally allocated for priority review applications. Dr. Gruber also said that FDA officials had opined that “absent a license, states cannot require mandatory vaccination” while raising concerns about the rise in COVID-19 cases.
“We too are concerned about the rising COVID-19 cases in the U. S. , however, our concern is that a review that is hyper-accelerated beyond the already very rapid September 15 target date and as a consequence, may be less thorough than our typical review seems more likely to undermine confidence in the vaccine (and, indeed, in FDA’s credibility) than to increase it,” Dr.
Gruber wrote. The lawmakers say the email “points to evidence” that the Biden administration sidelined experts and sacrificed “thoroughness and veracity” in approving the vaccine. “This is unconscionable,” they wrote.
In an FDA press release announcing the approval of the vaccine for those 16 and older, the agency said all approved vaccines “undergo the agency’s standard process for reviewing the quality, safety and effectiveness of medical products. ” “Our scientific and medical experts conducted an incredibly thorough and thoughtful evaluation of this vaccine. ” Dr.
Peter Marks, the director of FDA’s Center for Biologics Evaluation and Research, said in a statement. “The public and medical community can be confident that although we approved this vaccine expeditiously, it was fully in keeping with our existing high standards for vaccines in the U. S.
” For more information, visit The Washington Times COVID-19 resource page. • Joseph Clark can be reached at jclark@washingtontimes. com .
Copyright © 2023 The Washington Times, LLC. Click here for reprint permission . Please read our comment policy before commenting.
Click to Read More and View Comments Click to Hide.